Basic Reaction Mechanism
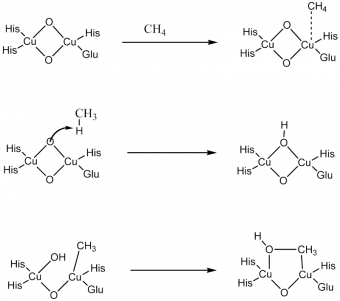
The first step in the reaction is for the hydrophobic pockets within the protein to direct the methane towards the active site. When the methane comes in contact with the copper a dative bond is formed between the methane and the oxygen. At this point there is homolytic cleavage of the carbon-hydrogen bond to form a radical CH3 species and the corresponding alcohol on the active site. This is followed by addition of CH3 back onto the copper site and the alcohol forming. These then form a bridging complex that reductively eliminates away to form methanol. The active site is regenerated using oxygen.
Construction of the Basic DiCopper-Oxygen Binding Site
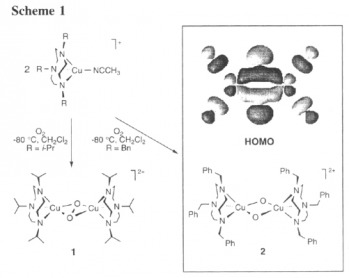
In 1995, Tolman et. al. constructed the first synthetic analogue of the proposed active site in pMMO. This showed the potential oxidation states and connectivity of the species.
This work lead to later work in terms of creating a workable synthetic analogue that would cleave the CH bond and form methanol in catalytic amounts.
(Tolman, J. Am. Chem. Soc. 1995, 117, 8865.)
This work lead to later work in terms of creating a workable synthetic analogue that would cleave the CH bond and form methanol in catalytic amounts.
(Tolman, J. Am. Chem. Soc. 1995, 117, 8865.)
Synthetic Molecule
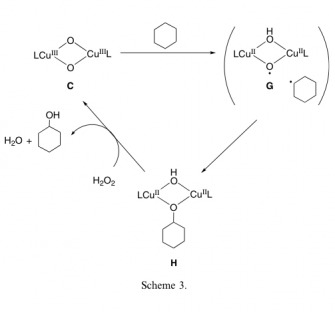
The Synthetic Analogue (C) was created in 2006 by Shinobu et. al.
The reaction consists of reacting cyclohexane with the catalyst and an equivalent of peroxide to produce cyclohexanol. This reaction proceeded at a reasonably efficient rate, producing the cyclohexanol as the major product and the cyclohexanone as the minor. This is important because the active site of pMMO also stops exclusively at the alcohol. At about an hour the total turnover number had reached 26, a fairly efficient and facile reaction.
The observed KIE for the reaction was 2.4, which suggests that the reaction is proceeding via the metal bound pathway as opposed to the free radical pathway. The active Copper site is regenerated with an equivalent of Hydrogen Peroxide.
This was the first oxidation of a CH bond that had been accomplished with a Copper center of this type. This proves invaluable to not only proving that the copper is a viable metal for the active site, but also what oxidation states are feasible as well.
The reaction consists of reacting cyclohexane with the catalyst and an equivalent of peroxide to produce cyclohexanol. This reaction proceeded at a reasonably efficient rate, producing the cyclohexanol as the major product and the cyclohexanone as the minor. This is important because the active site of pMMO also stops exclusively at the alcohol. At about an hour the total turnover number had reached 26, a fairly efficient and facile reaction.
The observed KIE for the reaction was 2.4, which suggests that the reaction is proceeding via the metal bound pathway as opposed to the free radical pathway. The active Copper site is regenerated with an equivalent of Hydrogen Peroxide.
This was the first oxidation of a CH bond that had been accomplished with a Copper center of this type. This proves invaluable to not only proving that the copper is a viable metal for the active site, but also what oxidation states are feasible as well.
The Identity of Ligand (L)
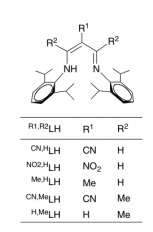
This ligand was synthetically created in the lab, the different substituents were present in the different starting materials. This ligand serves as a bidentate ligand, that was complexed to the Cu via refluxing in methanol.
The crystal structure for the metal-ligand complex was then obtained. It was found, in the reaction, that entries 1 and 2 yielded the best results in terms of forming the alcohol.
(Shimokawa, C.; Teraoka, J.; Tachi, Y.; Itoh, S. J. Ing. Biochem. 2006, 100, 1118-1127.)
The crystal structure for the metal-ligand complex was then obtained. It was found, in the reaction, that entries 1 and 2 yielded the best results in terms of forming the alcohol.
(Shimokawa, C.; Teraoka, J.; Tachi, Y.; Itoh, S. J. Ing. Biochem. 2006, 100, 1118-1127.)
Citations
Shimokawa, C.; Teraoka, J.; Tachi, Y.; Itoh, S. J. Ing. Biochem. 2006, 100, 1118-1127.
Tolman, J. Am. Chem. Soc. 1995, 117, 8865.
Yoshizawa, K.; Shiota, Y. J. Am. Chem. Soc. 2006, 128, 9873-9881.
