pMMO Crystal Structure for 3CHX
The second crystal structure was completed and published by Amy Rosenzweig et al. in 2008. The main difference was the protein was isolated from Methylosinus trichosporium OB3b which is a distinctly different organism. The structure was resolved to 3.9 angstroms with an R value of 0.342 and an R free value of 0.377. While many parts of the protein were found to be conserved, there are some significant changes in the second crystal structure. Two of the transmembrane helices are formed by peptides rather than attached to one of the main chains. Also, no zinc is found in this protein. All metal sites are copper and zinc was not required for crystallization. It was suggested by the authors of the paper that there may potentially be a dinuclear iron site found in the protein but was not present in the crystal structure.
pMMO Monomer
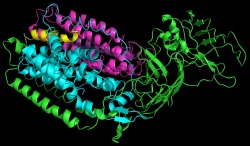
The monomoer obtained for the second crystal structure is very similar to that of the first. The overall architecture of the monomer is the same containing apha, beta, and gamma subunits. This crystal structure, however, showed two transmembrane helices that were small peptides.
pMMO Monomer with Metals
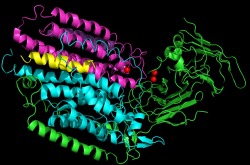
The metal centers associated with each monomer is also different from the previous crystal structure. This monomer contains one mononuclear copper site and one dinuclear copper site. The sites that were occupied by the zinc in the previous crystal structure, are occupied by copper in this crystal structure
Alpha Subunit
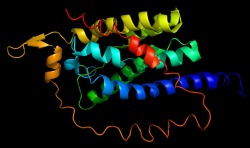
The alpha subunit is very similar to that of the previous crystal structure. It is composed of helices which will span the membrane. The helices are composed mainly of hydrophobic residues which embed in the plasma membrane
Beta Subunit
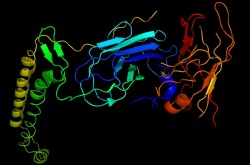
The beta subunit is very similar to the previous crystal structure. It contains the two beta barrels which make up the soluble region of the peptide as well as the catalytic site. Two alpha helices are also formed in the beta subunit that will embed in the plasma membrane.
Gamma Subunit
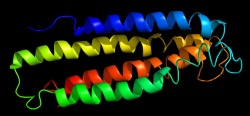
The gamma subunit is primarily composed of helices which span the membrane.
Twenty Amino Acid Peptide

One of the main structural differences between the crystal structures is the presence of two peptides that form part of the transmembrane domain. In the previous crystal structure there were no associated peptides. This peptide is made up for twenty hydrophobic residues
Twenty-Six Amino Acid Peptide

This peptide is the second peptide associated with the transmembrane region of the pMMO protein. This peptide is also only found in the crystal structure.
pMMO Trimer
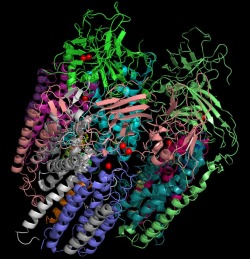
The crystal structures for the trimeric protein are generally in agreement. The main differences include six peptides in the transmembrane domain and only copper atoms found at the metal sites. This image depicts the trimeric protein with each chain a different color. The copper atoms are depicted as red spheres.
Citations
All images were obtained from the pdb file and manipulated in pymol
PDB file for 3CHX: http://www.pdb.org/pdb/explore/explore.do?structureId=3CHX
Hakemian, A. S., Kondapalli, K.C., Tesler, J., Hoffman, B. M., Stemmler, B.M., Rosenzweig, A.M., Biochemistry (2008) 47, 6793-6801
PDB file for 3CHX: http://www.pdb.org/pdb/explore/explore.do?structureId=3CHX
Hakemian, A. S., Kondapalli, K.C., Tesler, J., Hoffman, B. M., Stemmler, B.M., Rosenzweig, A.M., Biochemistry (2008) 47, 6793-6801
