pMMO Crystal Structure: 1YEW
The first crystal structure for pMMO was solved and published in 2005 by Raquel L. Lieberman and Amy Rosenzweig. The protein was isolated from Methylococcus capsulatus (Bath). The structure was refined to 2.8 angstroms with an R value of 0.273 and an R free value of 0.302. The protein is a trimer with three different metal sites in each monomer. Both copper sites are found in the soluble region of the protein and the zinc site is found the transmembrane region. There is some debate about whether zinc is actually present in the physiological protein. Zinc was required from protein crystallization.
pMMO Monomer
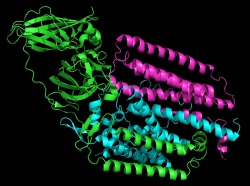
pMMO consists of three chains which make up a single monomer. Three monomers come together to form the trimer which creates a pore in the membrane. The methane enters the pore on the extracellular side of the membrane and is converted into methanol. The methanol then moves through the pore to the interior of the cell.
pMMO Monomer with Metal Centers
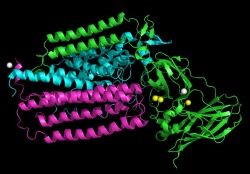
This image shows the pMMO monomer with the metal centers highlighted. The copper sites are displayed as yellow spheres. The zinc sites are displayed as grey spheres. This image shows that most of the metal centers are present in the extracellular end of the membrane.
Alpha Subunit
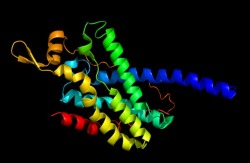
This image depicts the alpha subunit (pmoA) of the pMMO monomer. This chain consist mainly of alpha helices that will form the integral membrane domain of the protein.
Beta Subunit
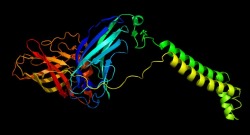
This image depicts the beta subunit (pmoB) of the pMMO monomer. This subunit makes up the extra cellular unit which contains the active site of the enzyme and two transmembrane helices.
Gamma Subunit
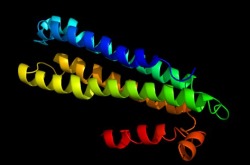
This image depicts the gamma subunit (pmoC) of the pMMO monomer. This subunit is also involved in forming the transmembrane helices of the protein.
Trimeric Structure
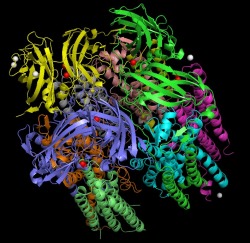
This image depicts the trimeric structure of pMMO with the metal centers displayed. Each subunit is displayed in a different color. The copper sites are colored red and the zinc sites are displayed as white. In the trimeric state, the protein is made up of a soluble region containing six beta barrel structures and a transmembrane region consisting of forty two helices. The metal centers consist of mononuclear copper sites, dinuclear copper sites, and mononuclear zinc sites.
Citations
All images were obtained from the pdb file and manipulated in pymol
PDB file for 1YEW: http://www.pdb.org/pdb/explore/explore.do?structureId=1YEW
Leiberman, R. L., Rosenzweig, A. C., Nature (2005) 434, 177-182
PDB file for 1YEW: http://www.pdb.org/pdb/explore/explore.do?structureId=1YEW
Leiberman, R. L., Rosenzweig, A. C., Nature (2005) 434, 177-182
